Turbidimetry and Nephelometry
When particles are suspended in a solution in a cuvette, they make the solution unclear (turbid). Incident light entering the cuvette will be subjected to three reactions;
1 some of the light will be absorbed (blocked) by the particles
2 some will be transmitted through the cuvette
3 some will be scattered in various directions.
Turbidimetry
Turbidimetry is involved with measuring the amount of transmitted light (and calculating the absorbed light) by particles in suspension to determine the concentration of the substance in question. Amount of absorbed light, and therefore, concentration is dependent on ; a) number
of particles, and 2) size of particles.
Measurements are made using light spectrophotometers
Clinical Applications
Determination of the concentration of total protein in biological fluids such as urine and CSF which contain small quantities of protein (mg/L quantities) using trichloroacetic acid
Determination of amylase activity using starch as substrate. The decrease in turbidity is directly proportional to amylase activity.
Determination of lipase activity using triglycerides as substrate. The decrease in turbidity is directly proportional to lipase activity.
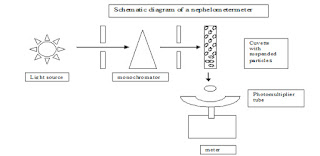
Nephelometry.
Principle
1. Nephelometry is concerned with measurement of scattered light from a cuvette containing suspended particles in a solution.
2. The components of a nephelometer are the same as a light spectrophotometer except that the detector is placed at a specific angle from the incident light.
3. The detector is a photomultiplier tube placed at a position to detect forward scattered light. Detectors may be placed at 90o, 70o or 37o depending on the angle at which most scattered light are found.
4. Since the amount of scattered light is far greater than the transmitted light in a turbid suspension, nephelometry offers higher sensitivity than turbidimetry.
5. The amount of scattered light depends on the size and number of particles in suspension.
6. For most clinical applications, the light source is a tungsten lamp giving light in the visible region
7. For higher sensitivity and for applications that determine the size and number of particles in suspension, laser light nephelometers is used.
Clinical applications of nephelometry.
Widely used to determine concentrations of unknowns where there is antigen-antibody reactions such as
Determination of immunoglobulins (total, IgG, IgE, IgM, IgA) in serum and other biological fluids
Determination of the concentrations of individual serum proteins; hemoglobin, haptoglobin, transferring, c-reactive protein, a1-antitrypsin, albumin (using antibodies specific for each protein)
Determination of the size and number of particles (laser-nephelometr}
Considerations in turbidimetry and nephelometry
1. The reaction in turbidimetry & nephelometry does not follow Beer's Law
2. Therefore, standard curves must be plotted and the concentration of the unknown is
determined from the standard curve.
3. Because the absorbance is dependent on both number and size of particles, the standard
solution which is used for the standard curve must have similar size in suspension as
unknown.
4. Because some precipitation and settlement of particles may occur with time, in order to
obtain good accuracy it is important to ; a) mix the sample well prior to placing the cuvette in
the instrument, and, b) keep the same time for measurement of every sample throughout the
measurement.
5. Kinetic reactions (measurement of the progress of reaction with time) provides higher degree
of accuracy, sensitivity, precision and less time than end-point reactions (measuring the
reaction at the start and finish of the reaction)
⦁ Additionally in kinetic reactions there is no need for reagent blank since the previous reading is taken as the base-line for the next reading.
⦁ Kinetic reaction may be taken in 60, 90 or 120 seconds (taking readings at 10 seconds intervals), whereas endpoint reactions may take much longer time e.g. 15 -120 minutes.
6. Selection of a wavelength
1. If both solution and suspended particles are colorless, then use any wave length in the visible range
2. If the solution is coloured but the particles are not coloured, then use a wave length that gives minimum absorption for the solution
3. If the particles are coloured and the solution is colorless then use a wavelength that gives maximum absorption with the particles
4. If both solution and particles are coloured then use two wavelengths; one that gives minimum absorbance for the solution and the other one maximum absorbance for the particles. Subtract the solution absorbance from the particles absorbance
















No comments:
Post a Comment